Small-angle scattering of proteins in solution.
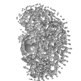 My friends sometimes jokingly refer to my research as ‘blob–ology’. To the untrained eye, the results of modeling our data actually do look like ‘blobs’.
My friends sometimes jokingly refer to my research as ‘blob–ology’. To the untrained eye, the results of modeling our data actually do look like ‘blobs’.
If we have been attentive to the preparation of our samples as well as to the data collection/analysis process, however, what these models represent, are the low-resolution shape of a folded, functional protein.
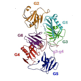
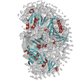
High-resolution structures of the same protein in similar buffer conditions (such as that from the x-ray crystal structure shown on the right here)should fit well within our small-angle scattering based models. (As seen in the figure to the left).
Why is shape important?
A protein is a polymeric molecule, a long chain of individual units linked covalently together. In the case of a protein polymer, it is of a specific length and unit sequence.
Every living system creates ~3000 different proteins, which function to do the chemical work required to sustain life.
For proteins to function, those chains must fold up into a unique, often globular, form.
It is the shape of a protein that dictates its function.
Sometimes protein function is regulated(turned on or off) by other molecules through shape changes.
The protein you see above is regulated by calcium ions, and its shape changes dramatically when calcium binds to it. We characterized this shape change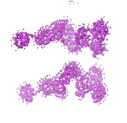 by small-angle scattering and published our results in the Journal of Biological Chemistry 2007.
by small-angle scattering and published our results in the Journal of Biological Chemistry 2007.
Our research will provide structural insights into the shape changes that are involved in activating or de-activating a protein. This regulatory process is referred to by biologists as ‘signal transduction’.
There are a myriad of signal transduction mechanisms that work to keep living systems alive.
Our scientific approach can provide a structural understanding of how a living system receives and responds to environmental information to elicit an appropriate response.