George Alyateem followed a modified expression and purification protocol for recombinant Human Gelsolin resulting in ~10 mgs of >95% purity. New lessons learned- 1) Don’t let the culture grow too long before innoculation: innoculate the culture when it has reached an OD600 of 0.5 with 1 mM IPTG then collect the cells at 3 hours post-induction. 2) Homogenize the cell lysate in order to shear the viscous nucleic acid contamination and avoid clogging up the spin column (benzonase was not required!). 3)Wash the Ni-resin bound protein with 25 mM Imidazole extensively (maybe even more than we did). Gelsolin will begin to elute in 50 mM imidazole but so does alot of other contaminating proteins. Best elution conditions for Gs is 100 – 150 mM imidazole.
Below is an SDS-PAGE gel of his HIS-tag affinity purification steps.
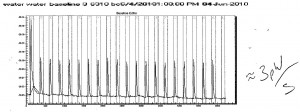
Runner up for Data of the week this week, is Brent Chesson’s ITC data. Brent improved his ITC technique consistently obtaining a 3 pW/s baseline (water-water titration).
He then tested his technique with an Acetic Acid titration experiment (self-designed). His analysis (still in process of perfection) gave a pKa of 4.618 which compares well with the literature value of 4.64! Lessons learned include: 1) de-gas all your solutions! 2) Load identical volumes in the sample and reference cells (300 uL min and 500 uL max) and 3) clean the cells well to get your baseline to 3 pW/s. [Both before and after your experiment]