Proteins are the functional products of gene translation and as such, structural analysis of proteins will be a primary objective for researchers in this genome-enabled era.
An important goal of such analysis is to discover how protein structures interact with one another to regulate specific cellular events.
Techniques capable of providing information on the resultant large macromolecular complexes will be a central component of any structural biologists’ toolbox.
The techniques of small-angle X-ray and neutron scattering on macromolecular complexes complement other structural approaches, providing a low-resolution framework from which to begin assembling molecular-level details on individual proteins or protein subunits into their intact, functional units.
 Indeed, interpretation of small-angle scattering data is most effective when used as a complementary tool with other structural and biochemical information such as that obtained from selected-site mutagenesis, circular dichroism, NMR, crystallography or electron microscopy, providing key pieces of information that complete a story.
Indeed, interpretation of small-angle scattering data is most effective when used as a complementary tool with other structural and biochemical information such as that obtained from selected-site mutagenesis, circular dichroism, NMR, crystallography or electron microscopy, providing key pieces of information that complete a story.
The biological application of small-angle scattering technologies, particularly that of neutron scattering using contrast variation, is relatively new and not yet widely explored; however, these methodologies show great promise in providing structural information on protein:protein interactions that can be difficult to obtain otherwise.
Evaluating Lipid Self-Assembly for membrane protein structural studies

ideal bicelle model
2012- present
Bicelles are water-soluble assemblies of lipids and detergents that are used to study membrane proteins, which is particularly useful for the development of drug delivery systems. Bicelles are formed from specific ratios of concentrations of the lipids (DMPC) and detergent (DHPC). This experiment used small-angle neutron scattering (SANS) to evaluate the effects of temperature and the lipid:detergent ratio on bicelle structure.
Protein Stabilization – Biotherapeutics
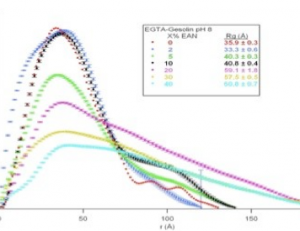
Gelsolin has immense potential for use as a biotherapeutic. We want to study the thermal and structural stability of gelsolin under varying environmental conditions for the purpose of developing a liquid formulation that could significantly increase its shelf-life.
Protein Stabilization – Specific Aggregation Studies
The function of the highly conserved protein, actin, is to polymerize or self-associate in a very specific way. We want to study the relationship between protein stability and aggregation, what better protein to study then one that is designed to self-associate.
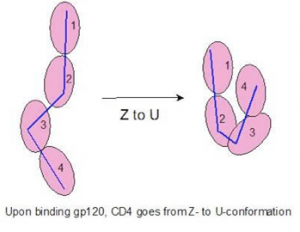
gp120 binding to CD4
The human HIV glycosylated viral coat protein, gp120, binds and recognizes the CD4 receptor protein on immunogenic T-cells. This binding event initiates viral entry into the host’s immunogenic cells.
Gelsolin Regulation of Actin
A major goal for my independent research has been to understand the regulatory mechanisms that govern the actin cytoskeleton by applying this “integration of structural biology tools” approach.