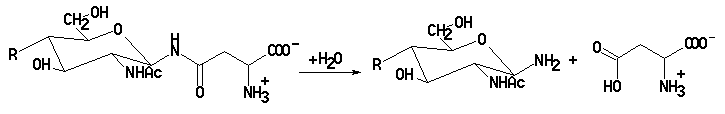Professor
Professor
Biochemistry
Burson 236
(704) 687-4844
jmrisley@uncc.edu
Associate Editor
Journal of Chemical Education
jrisley@jce.acs.org
B.S.: Ball State University, Muncie, Indiana
Ph.D.: Purdue University, West Lafayette, Indiana.
Post-doc: Purdue University, West Lafayette, Indiana
Risley Group Home Page
Publications
Courses Taught:
Advanced Biochemistry (CHEM 8165,CHEM 6165)
Biochemical Principles (CHEM 6101, CHEM 8101)
Special Topics in Biochemistry (CHEM 6060)
Special Topics in Biochemistry (CHEM 5090)
Biochemical Instrumentation (CHEM 4171)
Principles of Biochemistry (CHEM 4165, 4165L, 4166)
General Chemistry (CHEM 1203, 1203L, 1204, 1204L)
Chemistry in Today’s Society (CHEM 1111, 1112)
Laboratory in Chemistry (CHEM 1111L, 1112L)
Research Focus
I. Studies of Glycosylasparaginase, the Enzyme Involved in the Most Common Disorder of Glycoprotein Degradation
The catabolism of glycoproteins to the constituent amino acids and monosaccharides involves many different enzymes. While the enzymes that hydrolyze the peptide bonds in the polypeptide chain to amino acids show a generally broad activity toward different amino acid side chains, the enzymes that hydrolyze the bonds between the sugars in the carbohydrate moieties of glycoproteins generally have a high degree of specificity. A key enzyme in the catabolism of N-linked carbohydrate moieties is glycosylasparaginase, which hydrolyzes the amide bond between asparagine and N-acetylglucosamine to give aspartic acid and 2-acetamido-2-deoxy-b-D-glucopyranosylamine.
A decrease in activity of this enzyme gives rise to aspartylglycosaminuria, an inherited lysosomal storage disease, that leads to mental retardation and a shortened life span as the metabolite accumulates in cells, tissues, and body fluids. This disease has recently been recognized as the most common disorder of glycoprotein metabolism. There is no cure for the disorder. Mutations that give rise to the disorder have been elucidated and a crystal structure for the enzyme has been published. My lab is studying the fundamental physical and kinetic properties of the enzyme. We synthesize potential substrate analogues for the enzyme, inhibitors, possible suicide substrates, and transition-state analogues, and study their properties with the enzyme. We are also using molecular modeling of the enzyme in order to study various properties.
II. The 18O Isotope Shift in NMR.
NMR spectroscopy is a very important analytical tool in chemistry. It is used in almost all areas of chemistry, including analytical, biochemistry, inorganic, organic, and physical chemistry. One small area, and specialization, of study in NMR is the effect of isotopes on NMR active nuclei. Oxygen has three naturally-occurring isotopes, 16O, 17O and 18O; 16O is the most abundant at 99+%. Two NMR-active nuclei that are in important oxygen-containing compounds are 13C and 31P. The NMR signals of 13C and 31P have slightly different chemical shifts when bonded to 16O and 18O; the differences are very small – a few ppb (parts per billion) – but can be readily detected when the NMR spectrometer is correctly set up. We are using these 18O isotope shifts in 13C NMR and 31P NMR to study reactions and properties of molecules.