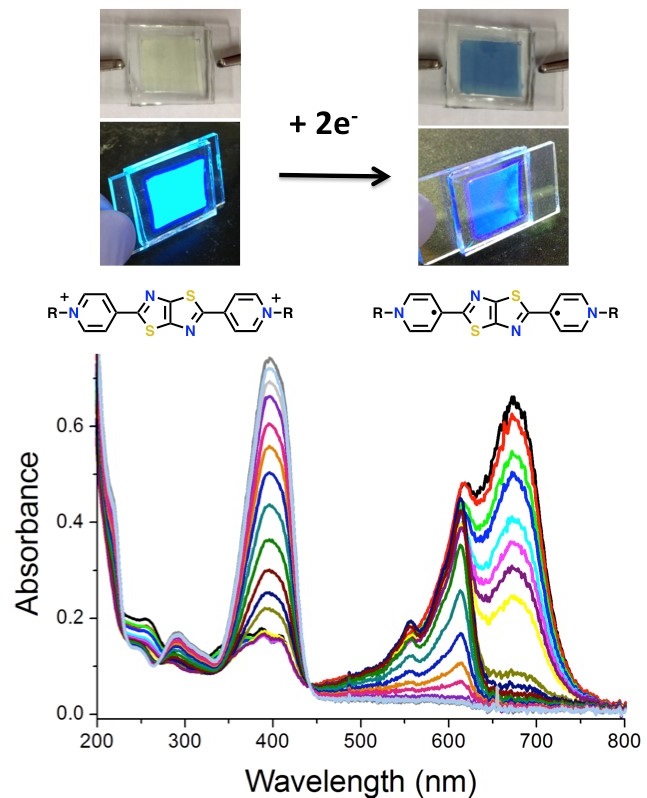
The purpose of this project is to study the fluorescent properties and photochemistry of thiazolothiazole viologens for solar energy applications. In particular we will study the photoinduced electron transfer events from a donor chromophore to an acceptor thiazolothiazole viologen (TTz). We will monitor these dynamics by studying the fluorescence both of the donor and acceptor molecules.
Research Mentors
Michael Walter (CHEM)
Description
Developing new methods to efficiently harvest solar energy and convert it into useable fuel is a great challenge of the 21st century. Using photocatalytic molecules to turn water and sunlight into valuable hydrogen “solar fuel” could enable solar energy to be stored and used on demand. The Walter research group has developed a new class of compounds that can be used to study photoinduced electron transfer events, using methods similar to those that utilize the electrochromic properties of methyl viologen. These thiazolothiazole viologen-like compounds show both electrochromism and strong fluorescence quenching after reduction. Using their unique properties, we can study the initial events that are critical for understanding light-induced catalytic events for the production of solar fuels. A variety of donor chromophore molecules will be studied such as porphyrins, ruthenium polypyridyl complexes, and phthalocyanines. The photophysics of thiazolothiazole compounds will be studied individually and as an acceptor species.
REU Students’ Role
The students in Dr. Walter’s lab will learn how to synthesize the TTz dye/electrochromic molecules that will be used to study photoinduced electron transfer. The photophysics of the TTz compounds will be characterized using time-resolved and steady-state photoluminescence measurements and UV-vis spectroscopy.